Spinning spider silk is now possible
Being able to produce artificial spider silk has long been a dream of many scientists, but all attempts have until now involved harsh chemicals and have resulted in fibers of limited use. Now, a team of researchers from the Swedish University of Agricultural Sciences and Karolinska Institutet has, step by step, developed a method that works. Today they report that they can produce kilometer long threads that for the first time resemble real spider silk.
The results were published in the journal Nature Chemical Biology.
Spider silk is an attractive material–it is well tolerated when implanted in tissues, it is light-weight but stronger than steel, and it is also biodegradable. However, spiders are difficult to keep in captivity and they spin small amounts of silk. Therefore, any large scale production must involve the use of artificial silk proteins and spinning processes. A biomimetic spinning process (that mimics nature) is probably the best way to manufacture fibers that resemble real spider silk. Until now, this has not been possible because of difficulties to obtain water soluble spider silk proteins from bacteria and other production systems, and therefore strong solvents has been used in previously described spinning processes.
Spider silk is made of proteins that are stored as an aqueous solution in the silk glands, before being spun into a fiber. Researcher Anna Rising and her colleagues Jan Johansson and Marlene Andersson at the Swedish University of Agricultural Sciences and at Karolinska Institutet have previously shown that there is an impressive pH gradient in the spider silk gland, and that this well-regulated pH gradient affects specific parts of the spider silk proteins and ensures that the fiber forms rapidly in a defined place of the silk production apparatus.
This knowledge has now been used to design an artificial spider silk protein that can be produced in large quantities in bacteria, which makes the production scalable and interesting from an industrial perspective.
"To our surprise, this artificial protein is as water soluble as the natural spider silk proteins, which means that it is possible to keep the proteins soluble at extreme concentrations", says Anna Rising.
To mimic the spider silk gland, the research team constructed a simple but very efficient and biomimetic spinning apparatus in which they can spin kilometer-long fibers only by lowering the pH.
"This is the first successful example of biomimetic spider silk spinning. We have designed a process that recapitulates many of the complex molecular mechanisms of native silk spinning. In the future this may allow industrial production of artificial spider silk for biomaterial applications or for the manufacture of advanced textiles", says Anna Rising.
Among the authors are also researchers from Donghua University (China), Universidad Politécnica de Madrid (Spain), University of Oxford (UK), KTH Royal Institute of Technology (Sweden), Uppsala University (Sweden) and Lund University (Sweden).
More information
Anna Rising, Senior Researcher
Dept. of Neurobiology, Care Sciences and Society
Karolinska Institutet, Stockholm, Sweden
&
Department of Anatomy, Physiology and Biochemistry
Swedish University of Agricultural Sciences, Uppsala, Sweden
+46 (0)8 585 853 78, +46 (0)70 974 48 88
email: anna.rising@slu.se or anna.rising@ki.se
http://ki.se/en/people/annris
OR
Jan Johansson, Professor
Dept. of Neurobiology, Care Sciences and Society
Karolinska Institutet, Stockholm, Sweden
+46 (0)8 585 853 78, +46 (0) 70 345 704 8
janne.johansson@ki.se
http://ki.se/en/nvs/portrait-of-professor-janne-johansson
Original reference
Marlene Andersson, Qiupin Jia, Ana Abella, Xiau-Yeen Lee, Michael Landreh, Pasi Purhonen, Hans Hebert, Maria Tenje, Carol V Robinson, Qing Meng, Gustavo R Plaza, Jan Johansson & Anna Rising. Biomimetic spinning of artificial spider silk from a chimeric minispidroin. Nature Chemical Biology, http://dx.doi.org/10.1038/nchembio.2269
Press images
(may be published without charge in articles about these findings, please acknowledge the photographer).

Figure 1. Biomimetic spinning of artificial spider silk.
a) Highly concentrated spider silk protein solution in a syringe is pumped through a pulled glass capillary with a tip size of 10-30 micrometre, with the tip submerged into a low pH aqueous collection bath. Fibers can be taken up from the collection bath (arrow) and rolled up onto frames. Photo: Marlene Andersson, Swedish University of Agricultural Sciences/Nature Chemical Biology
b) Photo of a fiber as it is spun in the low pH aqueous collection bath. Photo: Marlene Andersson, Swedish University of Agricultural Sciences/Nature Chemical Biology
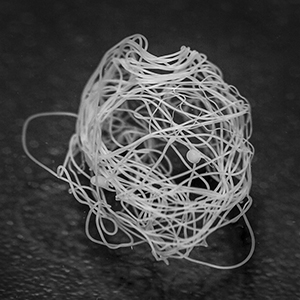
c) Wet fiber nest in low pH buffer. Photo: Lena Holm, Swedish University of Agricultural Sciences/Nature Chemical Biology
d) As-spun fibers on a frame. Photo: Marlene Andersson, Swedish University of Agricultural Sciences/Nature Chemical Biology
Fiber diameter in (b-c) is approximately 40 micrometre and in (d) 15 micrometre. Scale bar in (a) 3 cm, (b) 3 mm, and (c-d) 5 mm.

Figure 2. Anna Rising and Jan Johansson. Photo: Kerstin Nordling
Videos
Video 1. Formation of a continuous as-spun solid fiber of artificial spider silk in an acidic aqueous buffer collection bath. Published by: Anna Rising & Jan Johansson (Swedish University of Agricultural Sciences & Karolinska Institutet, Sweden). Source: Andersson et.al., Biomimetic spinning of artificial spider silk from a chimeric minispidroin, Nature Chemical Biology, http://dx.doi.org/10.1038/nchembio.2269.
Video 2. Artificial spider silk reeled onto a rotating frame in air. Published by: Anna Rising & Jan Johansson (Swedish University of Agricultural Sciences & Karolinska Institutet, Sweden). Source: Andersson et.al., Biomimetic spinning of artificial spider silk from a chimeric minispidroin, Nature Chemical Biology, http://dx.doi.org/10.1038/nchembio.2269.
Related references
Rising A,Johansson J. Toward spinning artificial spider silk. Nat Chem Biol. 2015. May; 11(15):309-15.
Andersson M, Chen G, Otikovs M, Landreh M, Nordling K, Kronqvist N, Westermark P, Jörnvall H, Knight S, Ridderstråle Y, Holm L, Meng Q, Jaudzems K, Chesler M, Johansson J, Rising A. Carbonic Anhydrase Generates CO2 and H+ That Drive Spider Silk Formation Via Opposite Effects on the Terminal Domains. PLoS Biol. 2014 Aug 5;12(8):e1001921
Kronqvist, N., Otikovs, M., Chmyrov, V., Chen, G., Andersson M., Nordling, K., Landreh, M., Sarr, M., Jörnvall, H, Wennmalm, S., Widengren, J., Meng, Q., Rising, A., Otzen, D., Knight, S. D., Jaudzems, K., Johansson, J. Sequential pH-driven dimerization and stabilization of the N-terminal domain enables rapid spider silk formation Nat Comm. 2014. 10(5):3254.
Wu S, Johansson J, Hovatta O, Rising, A. Efficient passage of human pluripotent stem cells on spider silk matrices under xeno-free conditions. Cell Mol Life Sci. 2015. Oct 1. doi:10.1007/s00018-015-2053-5.